The NCCN Guidelines® emphasize the importance of multidisciplinary consultation at a high-volume center, with appropriate interventions incorporated into the decision-making process for the diagnosis and management of BPDCN.
The following flowchart illustrates the NCCN Guidelines® recommendations for the diagnosis and management of BPDCN.
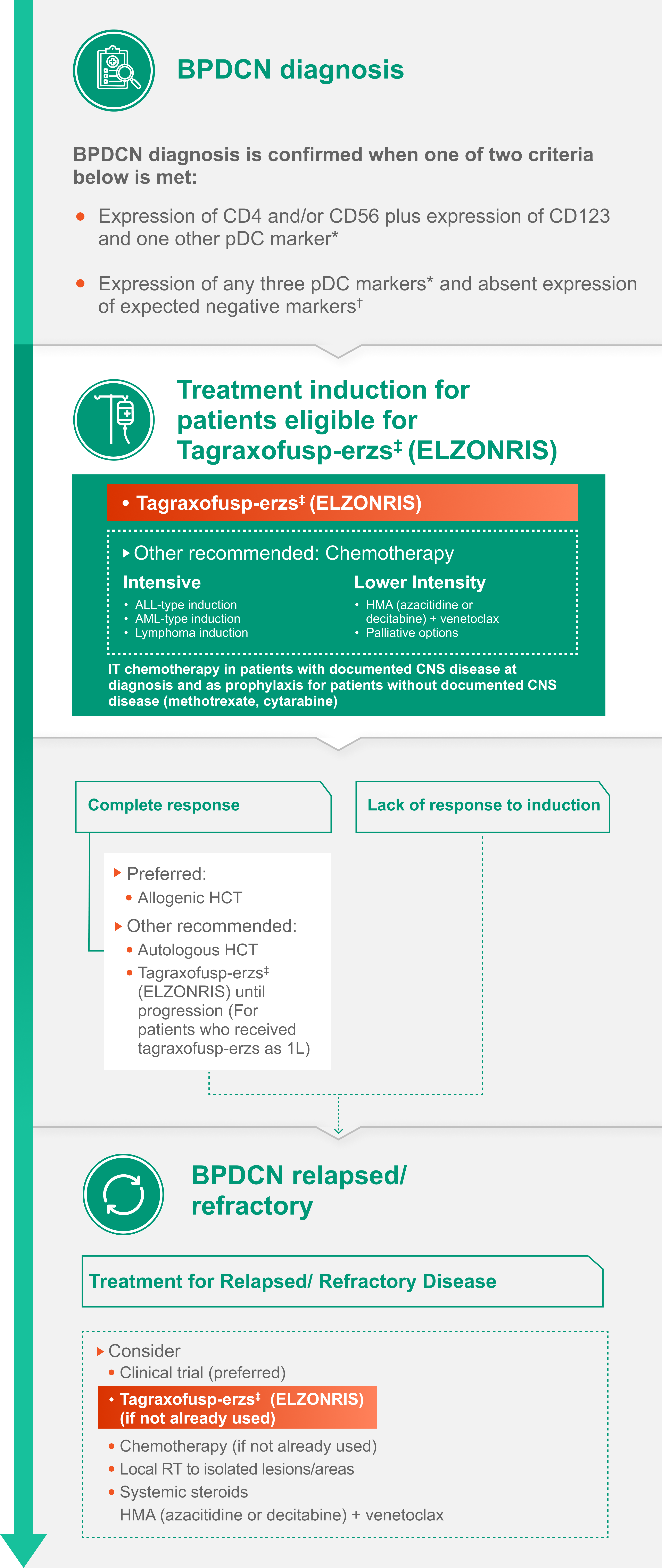
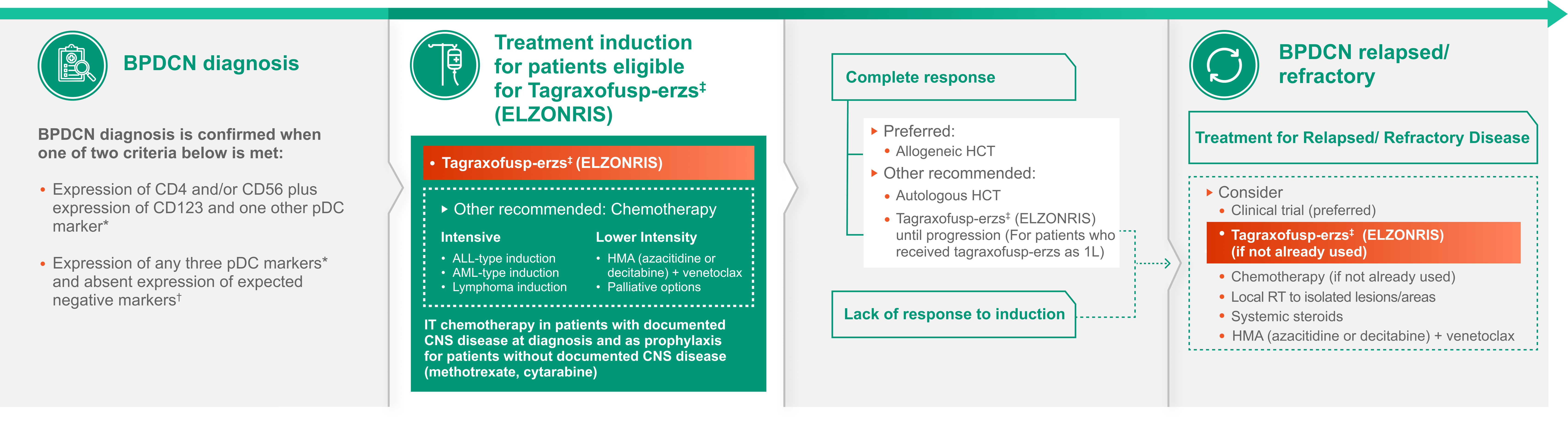
*pDC markers: CD123, TCF4, TCL1, CD303, CD304.
†Expected negative markers: CD3, CD14, CD19, CD34, lysozyme, myeloperoxidase.
‡Albumin ≥3.2 g/dL, LVEF ≥ institutional lower limit of normal, creatinine ≤1.5 mg/dL, bilirubin ≤1.5 mg/dL, AST/ALT ≤2.5 x ULN and no clinically significant cardiovascular disease.
This treatment sequence is for illustrative purposes only and is not comprehensive. Please refer to NCCN Guidelines for full treatment recommendations. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Abbreviations: 1L, first line; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BPDCN, blastic plasmacytoid dendritic cell neoplasm;
CD, cluster of differentiation; CNS, central nervous system; HCT hematopoietic cell transplantation; IT, intrathecal; LVEF, left ventricular ejection fraction; NCCN, National Comprehensive Cancer Network; R/R, relapsed/refractory; RT, radiation therapy; TCF4, transcription factor 4;
TCL1, T-cell leukemia/lymphoma 1; ULN, upper limit of normal.
- Reference:
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.3.2026. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed Dec 10, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way




