~60% to 90% of patients present with leukemic disease3,5
Important to know
- Primary sites of involvement include peripheral blood and bone marrow4,6
- Secondary sites of involvement include lymph nodes and viscera1,4
- In approximately 10% of cases, leukemic involvement may occur in the absence of skin disease7
- Comorbidities most commonly found are neutropenia, anemia, and thrombocytopenia1
- 20% of patients present with regional lymph node involvement6
- Neurological involvement of BPDCN is present in ~10% of patients at diagnosis and appears to be more frequent after relapse (~30%)8
- Cytology, in association with flow cytometry immunophenotyping and clinical history, can help obtain an accurate diagnosis of BPDCN9
BPDCN may be mistaken for other hematologic malignancies, such as AML, leukemia cutis, myeloid sarcoma, NK/T-cell lymphoma, ALL, MDS, CMML, and CTCL2-4,7
Historical overall survival10
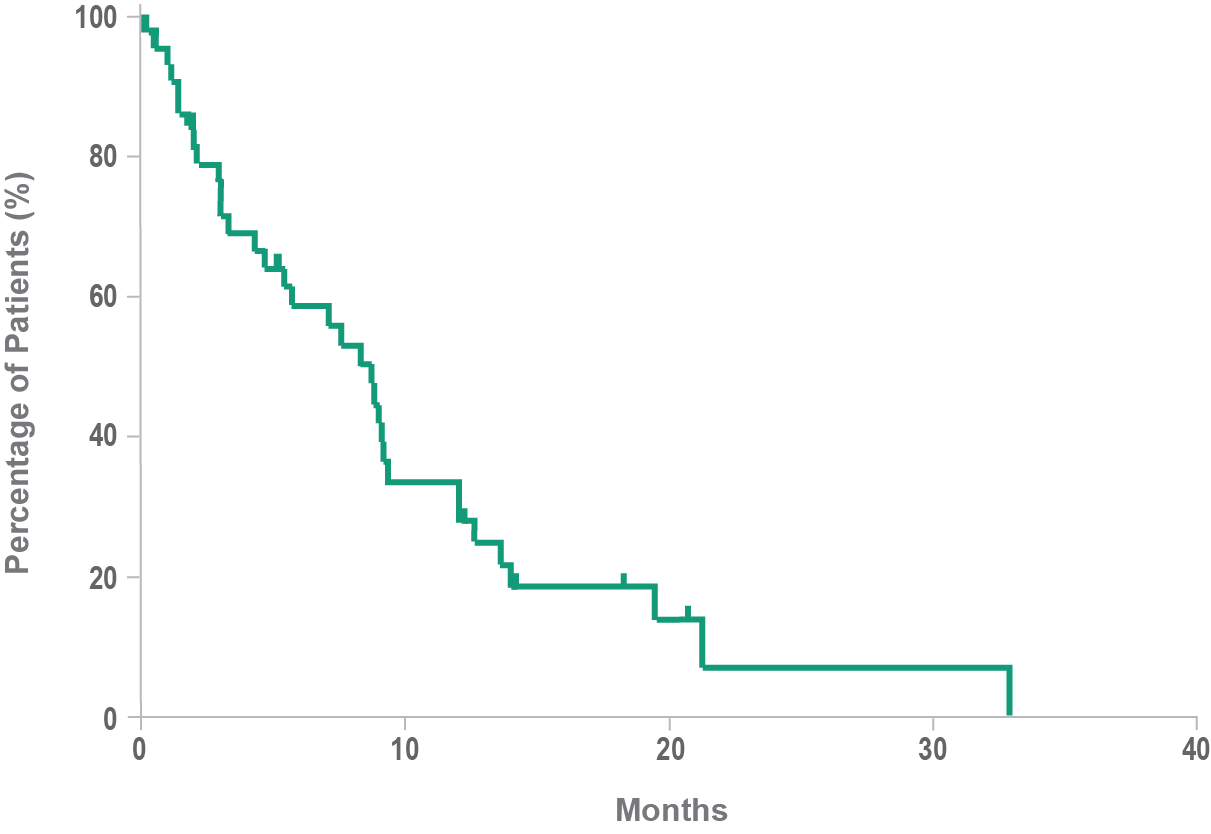
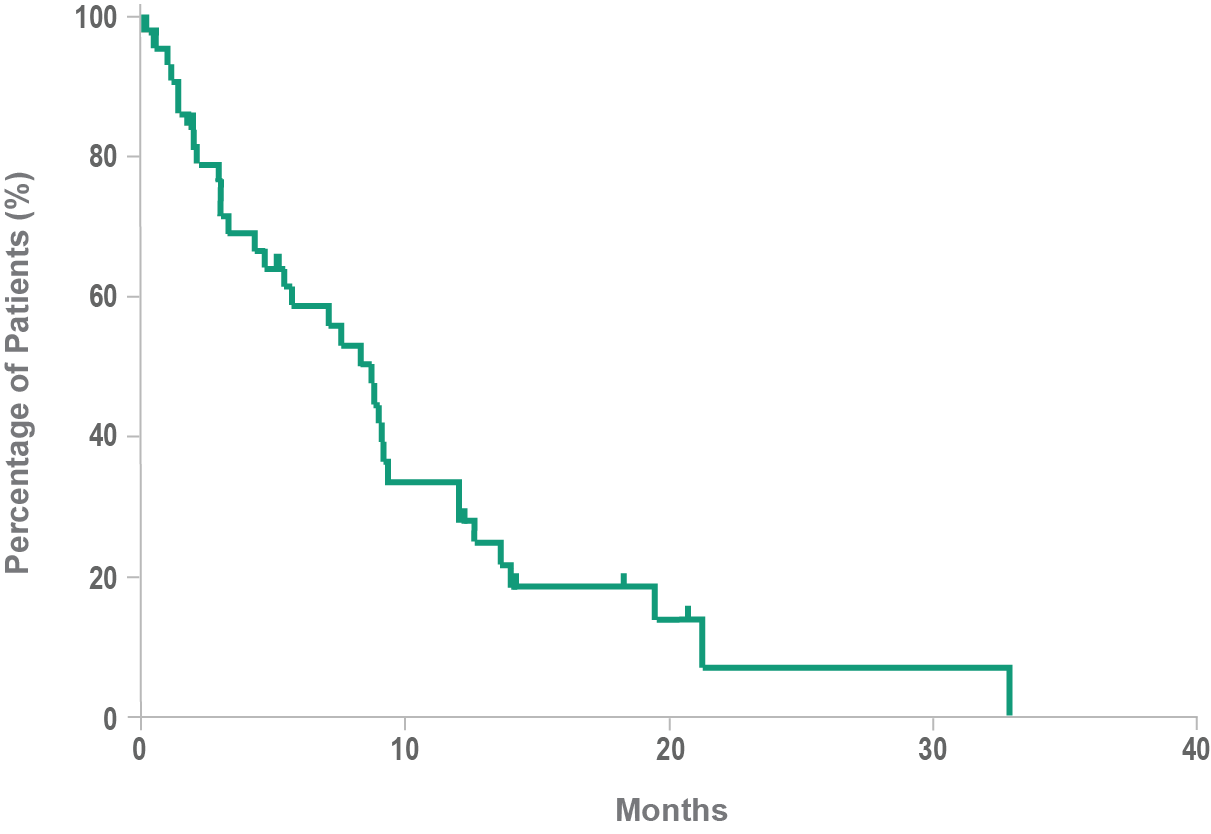
- Historically, median overall survival for BPDCN is approximately 8 to 14 months10,11
- BPDCN may rapidly progress to an aggressive leukemia4,10
- Following relapse, leukemic disease is often increasingly aggressive12
Early diagnosis of this aggressive hematologic cancer is essential1,7
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BPDCN, blastic plasmacytoid dendritic cell neoplasm; CMML, chronic myelomonocytic leukemia; CTCL, cutaneous T-cell lymphoma; MDS, myelodysplastic syndrome; NK, natural killer.
- References:
- Pagano L, et al. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016;174(2):188-202.
- Laribi K, et al. Blastic plasmacytoid dendritic cell neoplasm: from origin of the cell to targeted therapies. Biol Blood Marrow Transplant. 2016;22(8):1357-1367.
- Goyal A, et al. Blastic plasmacytoid dendritic cell neoplasm. In: Carter JB, et al, eds. Atlas of Cutaneous Lymphomas: Classification and Differential Diagnosis. Cham, Switzerland: Springer International Publishing Switzerland; 2015:193-203.
- Riaz W, et al. Blastic plasmacytoid dendritic cell neoplasm: update on molecular biology, diagnosis, and therapy. Cancer Control. 2014;21(4):279-289.
- Herling M, Jones D. CD4+/CD56+ hematodermic tumor: the features of an evolving entity and its relationship to dendritic cells. Am J Clin Pathol. 2007;127(5):687-700.
- Reichard KK. Blastic plasmacytoid dendritic cell neoplasm: how do you distinguish it from acute myeloid leukemia? Surg Pathol Clin. 2013;6(4):743-765.
- Hirner JP, et al. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34(3):501-509.
- Martín-Martín L, et al. Blastic plasmacytoid dendritic cell neoplasm frequently shows occult central nervous system involvement at diagnosis and benefits from intrathecal therapy. Oncotarget. 2016;7(9):10174-10181.
- Ferreira J, et al. Cytomorphological features of blastic plasmacytoid dendritic cell neoplasm on FNA and cerebrospinal fluid cytology: a review of 6 cases. Cancer Cytopathol. 2016;124(3):196-202.
- Pagano L, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98(2):239-246.
- Pemmaraju N. Novel pathways and potential therapeutic strategies for blastic plasmacytoid dendritic cell neoplasm (BPDCN): CD123 and beyond. Curr Hematol Malig Rep. 2017;12(6):510-512.
- Frankel AE, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014;124(3):385-392.




