- The study was designed as a nonrandomized, multistage, open-label, multicenter evaluation of ELZONRIS as monotherapy in patients with BPDCN, regardless of whether they had received previous treatment1,3
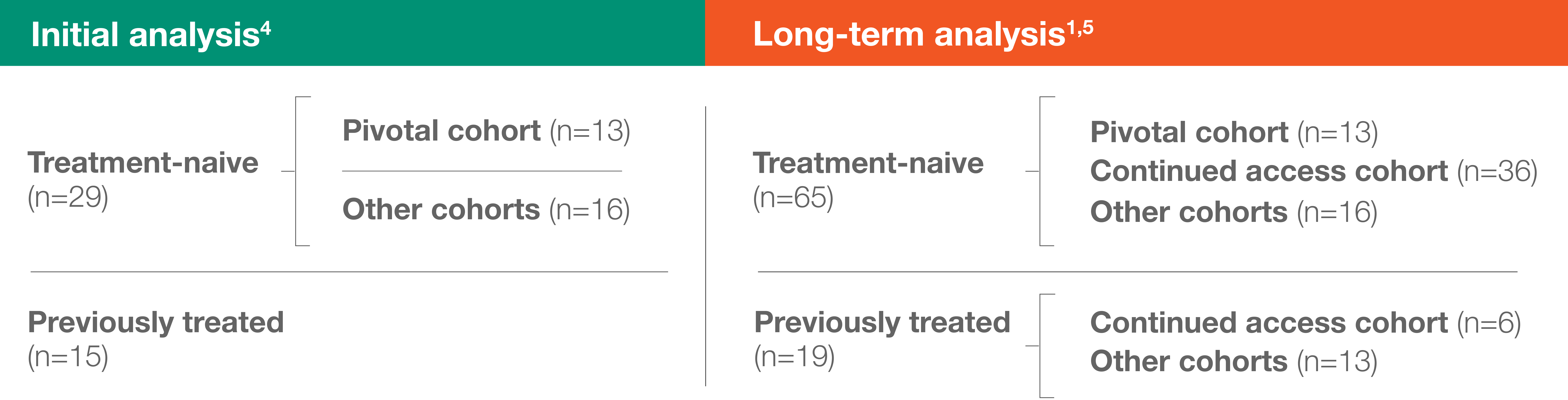

- FDA approval of ELZONRIS was based on the efficacy and safety observed in 13 treatment-naive patients in the pivotal cohort and 15 previously treated patients from other cohorts6
- Median follow-up was ~2 years in the initial analysis and ~3 years in the long-term analysis1,4
- The primary outcome was CR/CRc4*†
- The long-term analysis included all 44 patients from the initial analysis, as well as 40 others enrolled in the continued access cohort1,4,5
ELZONRIS 12 mcg/kg/day intravenous infusion on days 1 to 5 of a 21-day cycle4‡
- Repeat cycles until disease progression or unacceptable toxicity4
- Patients with CR/CRc received ELZONRIS until disease progression, unacceptable toxicity, or allogeneic or autologous SCT3,6
*CR criteria: normalization of blast percentage (≤5%) in the bone marrow; normalization of neutrophil count (≥1000/μL) and platelet count (≥100,000/μL) in the peripheral blood; absence of leukemic blasts in the peripheral blood; 100% clearance of all skin lesions from baseline; no new lesions in patients without lesions at baseline; regression of nodal masses to normal size on CT; no palpable nodules on the spleen or liver.3
†Criteria for CRc (CR with minimal residual skin abnormality): marked clearance of all skin lesions from baseline; residual hyperpigmentation or abnormality with BPDCN identified on biopsy (or no biopsy performed).3
‡Dosing period may be extended for dose delays up to day 10 of the cycle.6
Baseline patient characteristics (initial analysis)
Treatment-naive patients3,6
| Characteristic | Pivotal cohort (n=13) |
Other cohorts (n=16) |
All cohorts (n=29) |
|---|---|---|---|
| Male, % (n) | 84.6% (11) | 75.0% (12) | 79.3% (23) |
| Female, % (n) | 15.4% (2) | 25.0% (4) | 20.7% (6) |
| Median age (min, max) | 65.0 (22, 84) | 67.5 (28, 84) | 67.0 (22, 84) |
| ECOG PS, % (n) | |||
| 0 | 61.5% (8) | 43.8% (7) | 51.7% (15) |
| 1 | 38.5% (5) | 56.3% (9) | 48.3% (14) |
| BPDCN, % (n) | |||
| Skin | 100.0% (13) | 93.8% (15) | 96.6% (28) |
| Bone marrow | 53.8% (7) | 43.8% (7) | 48.3% (14) |
| Peripheral blood | 23.1% (3) | 25.0% (4) | 24.1% (7) |
| Lymph nodes | 46.2% (6) | 43.8% (7) | 44.8% (13) |
| Viscera | 15.4% (2) | 12.5% (2) | 13.8% (4) |
Previously treated patients3,4,6
| Characteristic | Previously treated patients (n=15) |
|---|---|
| Male, % (n) | 86.7% (13) |
| Female, % (n) | 13.3% (2) |
| Median age (min, max) | 72 (44, 80) |
| ECOG PS, % (n) | |
| 0 | 33.3% (5) |
| 1 | 66.7% (10) |
| BPDCN, % (n) | |
| Skin | 86.7% (13) |
| Bone marrow | 60.0% (9) |
| Peripheral blood | 6.7% (1) |
| Lymph nodes | 53.3% (8) |
| Viscera | 26.7% (4) |
| Prior treatments, % (n) | |
| Radiation therapy | 33.3% (5) |
| Stem cell transplantation | 26.7% (4) |
Previous lines of therapy: Previously treated patients4,6
| Number of lines | Previously treated patients |
|---|---|
| 1 | 60% (9) |
| 2-3 | 27% (4) |
| ≥4 | 13% (2) |
Baseline characteristics of treatment-naive patients (long-term analysis)1
| Characteristic | Long-term analysis (n=65) |
||
|---|---|---|---|
| Male, % (n) | 80% (52) | ||
| Female, % (n) | 20% (13) | ||
| Median age (min, max) | 68 (22, 84) | ||
| ECOG PS, % (n) | |||
| 0 | 48% (31) | ||
| 1 | 48% (31) | ||
| 2 | 3% (2) | ||
| BPDCN, % (n) | |||
| Skin | 92% (60) | ||
| Bone marrow | 49% (32) | ||
| Peripheral blood | 26% (17) | ||
| Lymph nodes | 51% (33) | ||
| Viscera | 14% (9) | ||
Baseline characteristics of previously treated patients (long-term analysis)1,3
| Characteristic | Long-term analysis (n=19) |
||
|---|---|---|---|
| Male, % (n) | 84% (16) | ||
| Female, % (n) | 16% (3) | ||
| Median age (min, max) | 72 (44, 87) | ||
| ECOG PS, % (n) | |||
| 0 | 37% (7) | ||
| 1 | 63% (12) | ||
| BPDCN, % (n) | |||
| Skin | 79% (15) | ||
| Bone marrow | 63% (12) | ||
| Peripheral blood | 5% (1) | ||
| Lymph nodes | 47% (9) | ||
| Viscera | 21% (4) | ||
| Prior treatments, % (n) | |||
| Radiation therapy | 32% (6) | ||
| Stem cell transplantation | 32% (6) | ||
BPDCN, blastic plasmacytoid dendritic cell neoplasm; CR, complete response; CRc, clinical complete response; CT, computed tomography; ECOG PS, Eastern Cooperative Oncology Group performance status; SCT, stem cell transplantation.
- References:
- Pemmaraju N, et al. Long-term benefits of tagraxofusp for patients with blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol. 2022;40(26):3032-3036.
- Study of tagraxofusp reports 90 percent response rate for deadly blood cancer with no prior available therapies. MD Anderson Newsroom. Published April 24, 2019. Accessed December 12, 2023. https://www.mdanderson.org/newsroom/study-of-tagraxofusp-reports-90-percent-response-rate-for-deadly.h00-159302256.html
- Data on file. Stemline Therapeutics, Inc.
- Pemmaraju N, et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2019;380(17):1628-1637.
- Pemmaraju N, et al. Long-term benefits of tagraxofusp for patients with blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol. 2022;40(26):3032-3036 [supplementary appendix].
- ELZONRIS [prescribing information]. New York, NY: Stemline Therapeutics, Inc.; July 2023.




